GOLDEN, Colo., March 7, 2014 – Researchers at Colorado School of Mines have discovered a breakthrough in the understanding of lithium ion batteries that may have important implications for future battery design. The research was recently reported in Nature Communications.
 |
| Ryan Richards |
 |
| Feng Lin |
“This research provides a nanoscale panorama for the ‘transformation’ of materials inside a lithium-ion battery electrode, which provides important knowledge to perceive battery lifetime and safety. With the knowledge obtained from this study, one can also improve battery materials design,” said Dr. Feng Lin, of Lawrence Berkeley National Laboratory (LBNL), a former PhD student of Mines Chemistry and Geochemistry Professor Ryan Richards. “This is actually how materials scientists work for most of time; we need to know exactly how materials perform in certain environments before searching for solutions to improve them. The functions of materials are usually controlled by the behaviors on the atomic scale, so leveraging fundamental knowledge with engineering is something we typically care about.”
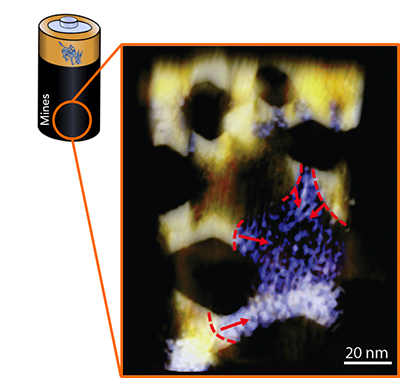
While lithium ion batteries are widely used, their overall efficiency, cyclability and capacity could be significantly improved if the phase conversion could be well understood. Using state-of-the-art electron spectroscopy and tomography as well as high-throughput synchrotron X-ray spectroscopy, the research group discovered and reconstructed three-dimensional heterogeneous charge distributions in a single battery particle.According to the researchers, the response of a material to phase conversion on the nanometer scale (10-9 m, 60000 times smaller than the diameter of a human hair) can directly affect its performance in a broad range of energy related applications including batteries, fuel cells and catalysts. Rapid development of energy storage technologies creates opportunities for a variety of emerging applications, including rechargeable batteries for electric vehicles, hybrid electric vehicles, plug-in hybrid electric vehicles, “thinner/smaller” consumer electronics and even large-scale grid electricity storage.
They also found that a heterogeneous phase conversion is dominant during the charging and discharging of NiO nanosheets in a lithium-ion battery, which is similar to the concept of heterogeneous nucleation in crystal growth. (The contours and arrows in the figure highlight the phase conversion fronts that had simultaneously propagated in a single NiO nanosheet.) This work overcomes a number of previous limitations that hindered the understanding of the charge distribution in lithium ion batteries.
“The fundamental insights reported in this work could only be attained by leveraging the collective expertise of all team members. The results are very exciting and I look forward to building upon this foundation with the team,” said Richards.
 The Richards group and Lin started collaboration with Dr. Dennis Nordlund and Dr. Tsu-Chien Weng of the SLAC National Accelerator Laboratory under a DOE funded project led by the National Renewable Energy Laboratory (NREL). Dr. Huolin Xin, an expert in electron spectroscopy and tomography at the Brookhaven National Laboratory (BNL), and Lin conceived and initiated the study and built a research team including Richards, Dr. Chunmei Ban of NREL, Dr. Dennis Nordlund and Dr. Tsu-Chien Weng of the SLAC National Accelerator Laboratory, and Dr. Ye Zhu of the Monash University, Australia.
The Richards group and Lin started collaboration with Dr. Dennis Nordlund and Dr. Tsu-Chien Weng of the SLAC National Accelerator Laboratory under a DOE funded project led by the National Renewable Energy Laboratory (NREL). Dr. Huolin Xin, an expert in electron spectroscopy and tomography at the Brookhaven National Laboratory (BNL), and Lin conceived and initiated the study and built a research team including Richards, Dr. Chunmei Ban of NREL, Dr. Dennis Nordlund and Dr. Tsu-Chien Weng of the SLAC National Accelerator Laboratory, and Dr. Ye Zhu of the Monash University, Australia.
"We are very excited to be part of this collaboration, combining development in nano-structured materials with state-of-the-art spectroscopic techniques and local expertise to address outstanding questions in energy sciences. We are uniquely positioned to make important findings in which details of the electronic and geometrical structure at various length-scales are important which are not limited to the application of battery anodes, but extends to many other fields, including current activities by Ryan's group in catalysis," said Nordland.
The Richards group is also working closely with these collaborators on probing the electronic structures at the surfaces of polar metal oxides in relevance to catalytic upgrading of low-carbon compounds. Sarah Shulda, a PhD student of Richards, is focusing her PhD career on this new collaboration.
The study used DOE user facilities including National Center for Electron Microscopy (LBNL), Center for Functional Nanomaterials at BNL and Stanford Synchrotron Radiation Lightsource (SLAC). A portion of the electrochemistry work was performed in Dr. Marca Doeff’s laboratory at LBNL. A portion of Xin’s TEM work was performed when he was a postdoctoral fellow in Dr. Haimei Zheng’s laboratory at LBNL.
Research in the Richards group at Mines has centered around nanoscale materials and their applications in renewable and sustainable energy. They have produced more than 100 papers, three books and seven patents since 2002.
Contact:
Karen Gilbert, Director of Public Relations, Colorado School of Mines / 303-273-3541 / kgilbert@mines.edu
Kathleen Morton, Communications Coordinator, Colorado School of Mines / 303-273-3088 / kmorton@mines.edu
